Objective
- Leigh Syndrome is a rare and devastating neurological disorder primarily affecting infants and children. The condition is characterized by progressive neurological deterioration, muscle weakness, and movement disorders, often leading to early death.
- The primary objective of this proposal is to develop a comprehensive model system for evaluating the therapeutic efficacy of potential treatments for Leigh Syndrome. This model system will serve as a valuable tool for preclinical research, enabling the rapid and accurate assessment of novel therapeutic interventions.
Methodology (Genetic modeling)
- Utilize induced pluripotent stem cells (iPSCs) derived from Leigh Syndrome patients or healthy controls as an in vitro model system.
- Employ mitochondrial DNA targeting base editing techniques, such as ABE (Adenine Base Editor), to introduce relevant mutations associated with Leigh Syndrome into the mitochondrial DNA (mtDNA) of iPSCs. And develop heart organoid or embryoid using iPSCs.
- Utilize state-of-the-art gene-editing techniques to introduce relevant mutations associated with Leigh Syndrome into animal genomes, particularly focusing on mitochondrial DNA (mtDNA).
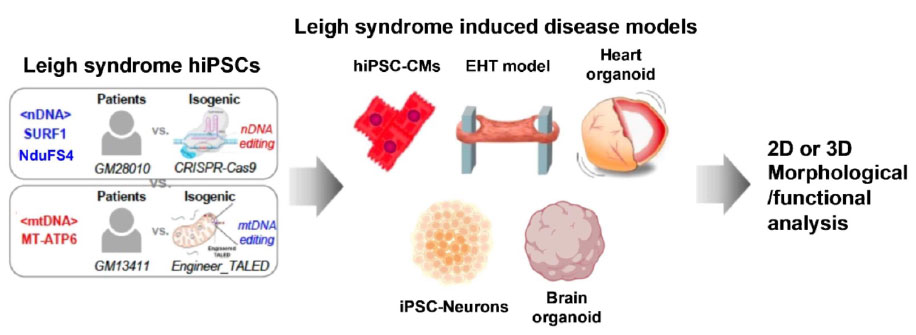
Phenotypic Characterization
- Characterize the genetic and phenotypic differences between mutant and wild-type iPSC lines, including mitochondrial function assays, gene expression profiling, and assessment of cellular bioenergetics, to validate the fidelity of the genetic modeling approach and confirm the recapitulation of Leigh Syndrome pathophysiology in vitro.
- Conduct detailed phenotypic analyses of the generated animal models to identify neurological, biochemical, and histological abnormalities characteristic of Leigh Syndrome. Utilize advanced imaging techniques, behavioral assays, and biomarker analysis to comprehensively assess disease progression and severity, taking into account the specific genetic modifications introduced via adenine base editing.
Efficacy Evaluation
- Systematically evaluate the efficacy of therapeutic interventions by monitoring changes in mitochondrial function, cellular viability, and disease-associated phenotypes in treated iPSC-derived cells compared to untreated controls.
- Efficacy of therapeutic interventions evaluated by monitoring changes in disease progression, muscle strength, neurological function, survival rates, and biochemical markers in treated animals compared to untreated controls.
Conclusion
- The development of a therapeutic efficacy evaluation model system for Leigh Syndrome represents a crucial step towards advancing treatment options for this devastating disorder.
Edgene’s proprietary technology
Mitochondrial A-to-G Editing in Mice
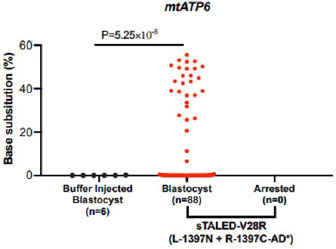
- Successfully introduced a Leigh Syndrome like point mutations In the mtATP6 gene in mouse embryos by sTALED.
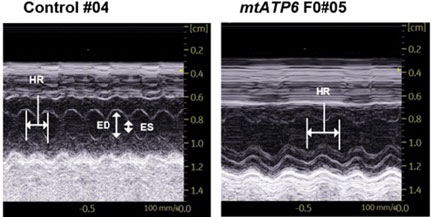
- Mice with mtATP6 mutations showed reduced heart rates
Pharmaceutical

